Introduction: Paroxysmal nocturnal hemoglobinuria (PNH) is a hematopoietic stem/progenitor cell disorder caused by PIGA mutations. All hematopoietic lineages derived from the affected clone exhibit the PNH phenotype [deficiency of glycosyl phosphatidylinositol linked membrane proteins (GPI-APs)]. Diagnosis is made by flow cytometric analysis of red blood cells (RBC), granulocytes, and monocytes. The percentage of granulocytes and monocytes with absent expression of GPI-APs is a measure of the size of the PNH clone. Typically, the percentage of GPI-AP deficient RBCs is somewhat less than that of granulocytes and monocytes due to selective destruction by complement-mediated intravascular hemolysis, manifested by high LDH, bilirubin, reticulocyte count and low haptoglobin. Herein, we report five patients with large PNH clones based on percentage of GPI-AP deficient neutrophils and monocytes, but with absent or near absent PNH RBCs and no biochemical evidence of hemolysis. We call this unique disease subtype, ahemolytic PNH.
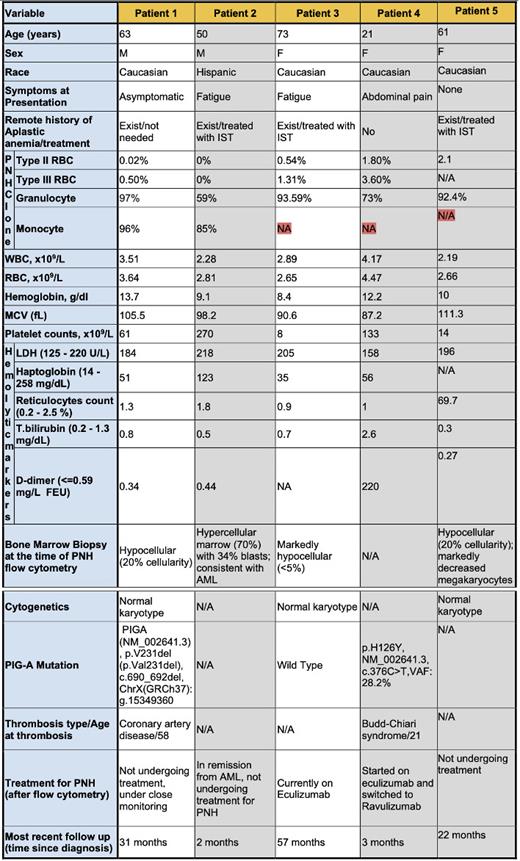
Methods: The medical records of PNH patients (174 patients with PNH granulocytes >20%) at the University of Texas Southwestern, Cleveland Clinic Foundation, and National Institutes of Health from 2000 to 2022 were examined. Information pertaining to their clinical, laboratory, and molecular attributes (NGS sequencing) was collected. Five patients with granulocyte clones >50% and RBC clones <5% were identified (Table).
Results: Patient 1 is a 63-year-old male with a remote history of non-severe aplastic anemia (AA) diagnosed at 11 years old. The first PNH flow cytometry, obtained at age 61 years, showed 0.02% type II PNH RBC, 0.5% type III PNH RBC, 96% PNH monocyte and 97% PNH granulocytes (Table). There was no biochemical evidence of hemolysis at the time of diagnosis or in the preceding 5 years. Patient 2 is a 50-year-old male who presented with pancytopenia and was diagnosed with acute myeloid leukemia with myelodysplastic-related mutations. Flow cytometry showed 85% PNH monocytes and 59% PNH with no detectable PNH RBCs and no biochemical evidence of hemolysis at diagnosis or in 3 years of follow-up (Table). Patient 3 is a 73-year-old female who presented with pancytopenia and marrow aplasia with unremarkable morphology, and a PNH granulocyte clone of 93.6% with 1.3% type III PNH RBCs and 1.3% and 0.5% type II PNH RBCs, with no evidence of hemolysis (Table). Patient 4 is a 21-year-old female who presented with Budd-Chiari syndrome. Flow cytometry showed 73% PNH granulocytes with 3.6% type III PNH RBCs and 1.8% type II PNH RBCs. There was no biochemical evidence of hemolysis, but because of thrombosis, she was treated complement inhibitor therapy (Table). Patient 5 is a 61-year-old female with history of severe aplastic anemia treated with immunosuppression (IST) and eltrombopag. PNH flow cytometry showed PNH granulocytes of 92.4% and 2.1% PNH type II RBCs 2.1% post IST treatment with no biochemical evidence of hemolysis (Table).
Conclusion:
Herein, we present five patients with a distinct PNH phenotype that we call ahemolytic PNH. Except for the absence or near absence of PNH erythrocytes, this entity shares clinical and pathophysiological features with classic PNH, including thrombophilia (Budd-Chiari Syndrome, Table). The basis of this phenotype is speculative but may be determined by the genotype of the affected HSPC in which the somatic mutation of PIGA first occurred. According to this hypothesis, somatic mutations other than PIGA skew differentiation toward granulocyte/monocyte lineages.
The International PNH Interest Group recognizes the following three categories of PNH: classic PNH, PNH in the setting of another define bone marrow abnormality, and subclinical PNH. Like ahemolytic PNH, subclinical PNH has no biochemical evidence of hemolysis. In this case, however, the absence of hemolysis is due to the small size of the PNH clone (median clone size <1%) as determined by flow cytometry analysis of peripheral blood granulocytes and monocytes. We propose that ahemolytic PNH, defined as patients having less than <5% PNH red cells and >50% PNH granulocytes/monocytes, be recognized as a distinct category of PNH.
Disclosures
Maciejewski:Alexion: Membership on an entity's Board of Directors or advisory committees; Regeneron: Consultancy, Honoraria; Novartis: Honoraria, Speakers Bureau; Omeros: Consultancy. Bat:Alexion pharma: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal